Heat of Combustion Formula
It can be determined by subtracting the latent heat value of water vaporization formed in the reaction from the gross calorific value which is the higher heating value. The effective heat of combustion h c eff is obtained by multiplying equation 1 by the combustion efficiency in the flame χ h c eff χ h cv.

Enthalpy Heat Combustion Experiment 4 Energy Level Heat Exothermic Reaction
Char respectively and µ is the char fraction.

. Kgcal Conversions 1 kgcal 41868 kJkg 18 Btulbm. Combustion Formula The heat produced during combustion Heat of formation of the products Heat of the formation of reactants. The heat of combustion is calculated using the heat of combustion equation.
This chemistry video tutorial explains how to calculate the enthalpy change of a reaction using the enthalpy of formations found in the appendix section of y. Organized by textbook. The heat of combustion of octane C8H18 Mm 114 gmol is -5500 kJmol.
The products of the incomplete combustion of. 85 rows The molweight of ethanol is 21201 6101 11600 4608 gmol The heat of combustion of ethanol ΔHc C2H6O l 136691 kJmol 1000 gkg 4808 gmol. Uses Hesss law to show how heat of com.
These are then summed. The heat of combustion is the heat produced when one mole of a substance is completely burnt in oxygen under standard conditions. The molar heat of combustion H e is the heat released when one mole of a substance is completely burned.
This formula is based on the calorimeter study and has as important parameters the mass of water. Next you need values for the heat of reaction for oxidation of each constituent to CO 2 and H 2 O. What is the incomplete combustion of octane.
Formula for Combustion Click Here for Sample Questions The heat produced during combustion is equal to the product formation heat minus the reactant formation heat. The substances can be elements or. Working with different elements and different forms means youll need to convert the heat of combustion to and between units.
Up to 24 cash back Heat of combustion per gram -1367460 -297 kJg Specific heat example. The complete Combustion process is oxidation of. Typical combustion reactions involve the reaction of a carbon.
First you need the composition of the supplied gas. Calculate the energy required to heat 120mL of water for a cup of coffee to boiling.

Enthalpy Heat Combustion Experiment 4 Energy Level Heat Exothermic Reaction
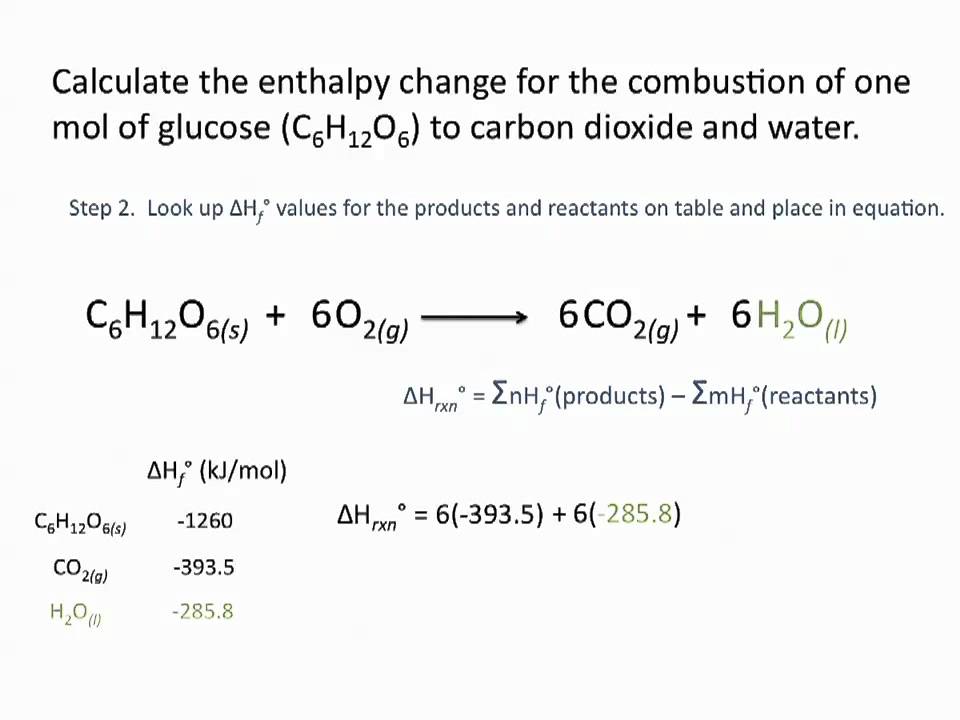
Enthalpies Of Formation Chemsitry Tutorial Science Chemistry Chemistry Tutorial

Combustion Reaction Definition And Examples Reactions Chemical Reactions Chemical Bond

Comments
Post a Comment